Mechanisms and consequences
The Zinc Switch
The zinc switch is an excellent example of an albumin-based drug-nutrient interaction mechanism.
Essentially the zinc switch is the occupancy or allosteric change to FA2 binding sites causing zinc exclusion from MBS-A. Zinc exclusion includes both eviction from and inhibited uptake by MBS-A. MBS-A is also known as Multi-metal Binding Site A, Site A, Cadmium site A.
The zinc switch is located in the domain I/II interface on albumin.
FA2 (Fatty Acid Binding Site 2)
FA2 is located in the domain I/II interface, and overlaps the MBS-A site.
Albumin is the primary carrier for NEFAs (non-esterified fatty acids) which are free (unbound), typically long chain fatty acids such as palmitate and stearate. Fatty acid carbon chain length and saturation inversely correlate with zinc binding to MBS-A.
FA1, FA2 and FA7 are functionally and allosterically. Consequently, binding on one site is likely to alter binding capabilities (competitive and/or allosteric) of the other two sites.
Drugs that bind to -
- FA2 include – cannabis (primary binding site), ibuprofen (tertiary binding site), warfarin (tertiary binding site);
- FA1 include - rifampicin, isoniazid, fipronil;
- FA7 include – azapropazone, diclofenac, diflunisal, gemfibrozil, ibuprofen, indomethacin, phenylbutazone, tolbutamide, warfarin.
As fatty acid levels increase FA2 occupancy, zinc binding capacity to albumin decreases.
Zinc
Albumin regulates zinc levels by being both its buffer/chelator and a systemic (throughout the body) transporter.
Zinc’s binding sites on albumin include –
- primary – MBS-A,
- secondary – MBS-B,
- tertiary – currently 6 known sites.
The alternate sites (secondary, tertiary) extend zinc’s binding capacity when zinc levels are elevated or MBS-A binding is compromised.
Zinc binding to albumin is dependent upon the concentration of free zinc (zinc ions) in the blood. Increasing free zinc levels means decreasing zinc binding capacity to albumin.
The main consequences of an increase in free zinc levels in blood include –
1) redistribution of zinc to other plasma proteins such as insulin and HRG (histidine-rich glycoprotein),
2) removal of zinc from the plasma.
Excessive free zinc levels in the plasma are toxic.
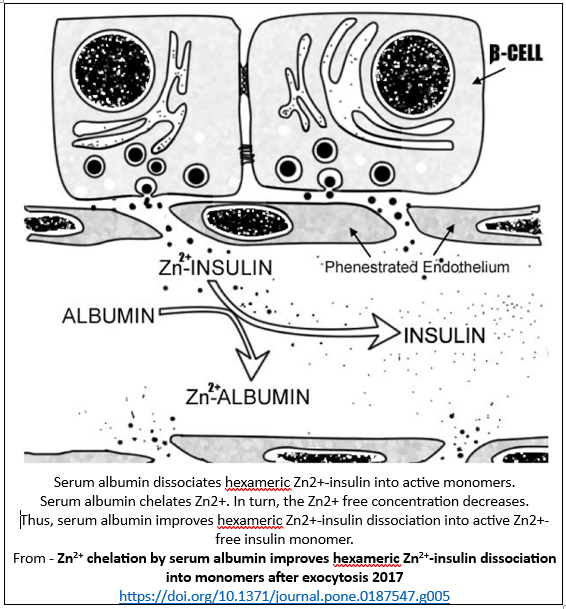
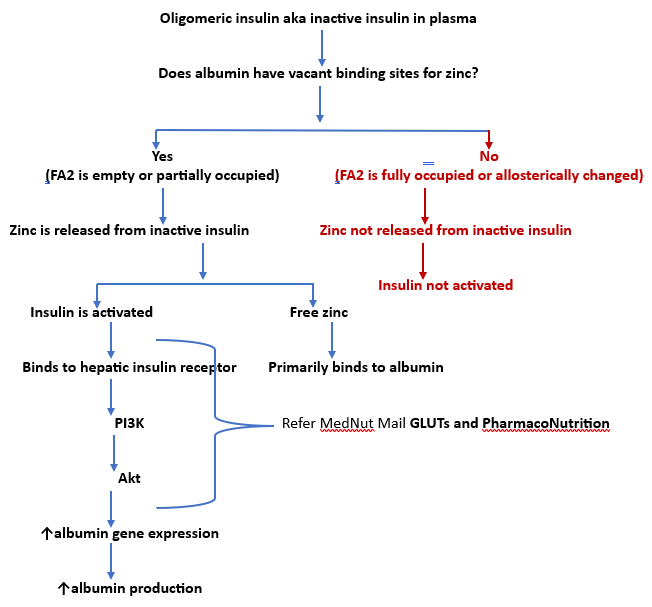
Albumin and insulin
Insulin is released from the pancreas into the plasma as a hexamer comprising 6 insulins bound by 2 zincs. The release of zinc from hexamers produces insulin monomers and oligomers (dimers, trimers, tetramers, hexamers). Only insulin monomers are biologically active.
Plasma levels of monomers and oligomers are fluid and indicate zinc’s addition or removal from the oligomers. Insulin fluidity is typically influenced by environmental factors such plasma zinc levels. As zinc levels increase, the levels of oligomers increase via a monomer-dimer-tetramer-hexamer equilibrium.
Albumin is dependent upon insulin for its production via increased gene expression. Insulin activation is dependent upon albumin accepting its cleaved zincs. Albumin and other zinc binders do not modify insulin release.
Albumin and coagulation
Early evidence indicates the Zinc Switch may be important in the coagulation cascade at injury sites. Evidence indicates excess zinc –
- can bind to HRG which is a multi-function plasma protein that regulates coagulation;
- increases turbidity which reflects the size and/or density of the clot formed. Fatty acid levels also correlate with turbidity.
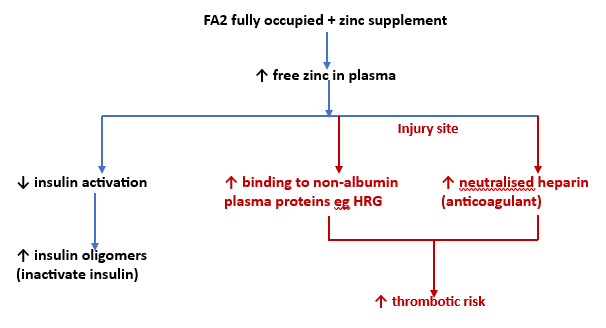
Zinc Switch and zinc supplements
Increased zinc binding to non-albumin plasma proteins may cause an apparent “mild zinc deficiency”. The initial research is based on coagulation responses in those with diabetes. However, a particular concern is the effects of zinc supplements when FA2 is fully occupied.
Concerns
Very limited evidence indicates fully occupied FA2 means the other 5 minerals bound to MBS-A are also excluded.
Stuff we really need to know–
- the clinical significance of FA1 and FA7 allosteric impact on FA2, and MBS-A consequences;
- identification of all the prescribed medicines, including their doses that directly impact FA2, FA1, FA7.
Allostery adds an extra layer of complexity, and interest, to albumin-based drug-nutrient interactions.
Clinical Questions
When you a review a person prescribed medicines that directly or indirectly affect the zinc switch, would you question -
- whether they are altering albumin’s capacity to carry zinc and the other MBS-A-bound minerals thus altering their availability?
- an apparent “mild zinc deficiency” and clarify adequacy of zinc intake before considering zinc supplements?
Conclusions
The zinc switch refers to an albumin-based drug-nutrient interaction mechanism involving zinc exchange. If the exchange does not proceed then outcomes include poor glycaemic control and likely altered coagulation.
Please read this as it is important …
The information in this article is provided to support Health Professionals. It is not an exhaustive protocol and Health Professionals are advised that adequate professional supervision is accessed to ensure that Duty of Care obligations with respect to safe administration of medicines is met for each consumer.