Albumin’s haem site is important in regulating free haem levels within our blood.
Mechanisms and consequences
Haem sources
Free haem sources include newly-produced haem for integration into haemoproteins, and recently released haem from haemoproteins.
Factors that increase haem levels include increased haem production, increased haemoprotein breakdown, and impaired haem uptake.
Haem’s functions
Haem’s functions include -
- oxygen transport in the blood utilising haemoglobin;
- oxygen storage in the tissues utilising myoglobin;
- producing cytochrome P450 enzymes responsible for the metabolism of external substances;
- immune function processes including immune-receptor sensing;
- red blood cell activation and regulation;
- inducing cell signalling and sensing pathways;
- modifying oxidative stress by sequestering active iron before it causes reactive oxygen species to form.
Excessive haem
Excessive free haem is extremely toxic for cells, mostly resulting in cell death, as it -
(i) disrupts the lipids in the cell membranes;
(ii) has pro-inflammatory properties;
(iii) impairs the functions of blood vessels;
(iv) causes oxidative damage to cells.
Consequently, haem levels are very tightly regulated to optimize cellular function and minimize negative physiological impacts.
Regulatory systems
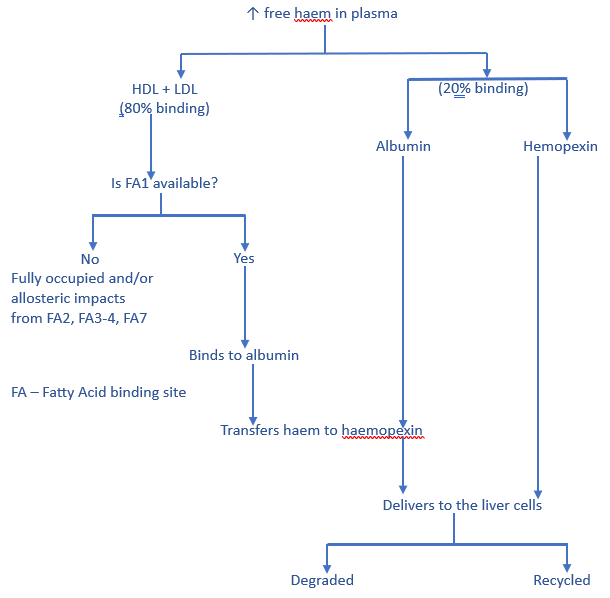
Albumin’s contribution to haem regulation is to bind and remove excess amounts from the blood.
Excessive haem levels are also managed by their binding to high-density lipoproteins (HDL), low-density lipoproteins (LDL), hemopexin, and albumin. The plasma initially binds 80+% of the haem to HDL and LDL, and the remainder to hemopexin and albumin. Albumin then transfers the haem from HDL and LDL to hemopexin for delivery to the liver for degradation or recycling.
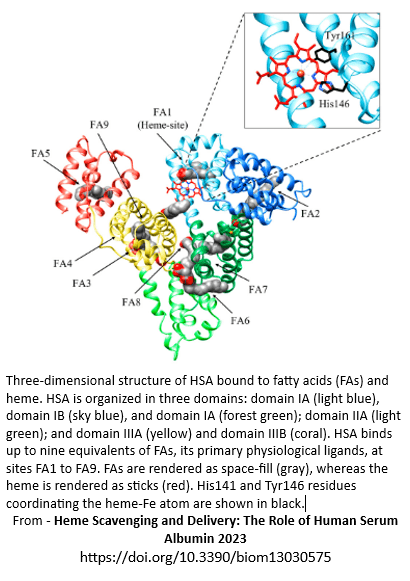
Haem site
Haem site is also known as the haem binding pocket, haem pocket, FA1 (fatty acid binding site 1) and is located in subdomain IB.
Some fatty acids, bilirubin, biliverdin, haem, hemin, methyl orange and several pharmaceuticals can be carried on the haem site.
FA1, FA2, FA3-4, and FA7 are functionally and allosterically linked. Consequently, binding on one site is likely to alter binding capabilities on the other sites. Ultimately allosteric impact may not be a straightforward association. Some evidence indicates drug-induced allosteric inhibition of haem binding to albumin only occurs when albumin levels are low.
Haem binding to albumin is altered by, or can inhibit, compounds binding to the FA2, FA3-4 and FA7 sites. For example, substances binding to albumin’s site on –
- FA1 - directly impair haem binding,
- FA7 - allosterically decrease haem binding.
Clinical Concerns
The research on allosteric impacts on albumin’s carrying capacity of nutritional factors is remarkably limited. This inadequacy of evidence means optimization of the pharmaceutical therapeutic benefits cannot be realized.
Clinical Questions
When you a review a person’s prescribed medicines that directly or indirectly affect the haem site, would you question -
- whether they are also altering albumin’s capacity to carry the Mineral binding site-A (MBS-A)-bound nutrients (calcium, cobalt, copper, magnesium, manganese, zinc) thus altering their availability?
Conclusions
Albumin’s haem site is important in a range of physiological functions, and its alteration can have significant nutritional consequences.
Please read this as it is important …
The information in this article is provided to support Health Professionals. It is not an exhaustive protocol and Health Professionals are advised that adequate professional supervision is accessed to ensure that Duty of Care obligations with respect to safe administration of medicines is met for each consumer.