Mechanisms and consequences
Albumin and the fatty acids (FAs) interactions have an important physiological relationship. Albumin is a key carrier for many of the fatty acids, normally binding up to 99% non-esterified fatty acids (NEFAs). NEFAs are free (unbound), typically long chain, fatty acids. The binding affinities of fatty acids are dependent upon carbon chain length.
Types of binding
When binding to albumin, fatty acids alter its structure allosterically. This means fatty acid binding at X site alters what (other substances) can be carried at Y site.
Substances are not able to simultaneously occupy the same binding site on albumin.
Factors that can alter fatty acids binding to albumin include dietary fat (type and quantity), and some prescribed medicines.
The binding on albumin can be co-operative or competitive.
Co-operative binding
Occurs when both compounds are able to interact with albumin simultaneously.
Co-operative binding can also be allosteric. For example, Site I (Sudlow Site 1, subdomain IIA) and Heme site (FA1, subdomain IB) are allosterically coupled. Consequently, Site I compounds may affect the bindings of Heme site compounds with albumin and vice versa.
Co-operative binding can cause changes in binding affinity.
Competitive binding
Compounds cannot be bound simultaneously if they both bind to the same binding site or their binding sites overlap. For example, α-linolenic acid binds to FA7 (subdomain IIA) and warfarin binds to Site 1 (subdomain IIA). FA7 overlaps Site 1 so when both want to bind at the same time the binding is competitive.
When compounds compete for the same binding site, the outcome is determined by their concentrations and affinities. For example, α-linolenic acid has a higher affinity for albumin than warfarin.
Fatty acid binding-induced changes
Fatty acid binding causes changes to albumin’s structure (shape change) and consequently function.
Structural/shape change
Albumin is quite flexible structurally in order to accommodate the various shapes and sizes of its load.
Examples of fatty acids changing albumin’s shape -
- binding of fatty acids cause Site 1 (subdomain IIA) to extend significantly beyond subdomain IIA;
- binding of long chain fatty acids causes domains I + III to rotate relative to domain II;
- FA1 (subdomain IB) partially overlaps with haem site (subdomain IB). Consequently, haem and some fatty acids can change the shape of subdomain IB;
- pocket accommodation of fatty acids in FA2 (subdomains IA + IIA interface) requires domain I rotation relative to domain II;
- binding of fatty acids can alter the volume of the FA7 (subdomain IIA) binding site;
- FA9 (subdomains IA-IB-IIA + IIB-IIIA-IIIB interface) is formed by a fatty acid inducing structural change.
The consequences of changes in the albumin structure impacts various –
- nutrients - by altering their availability;
- drugs – by ultimately affecting their availability and/or effectiveness.
Changing function
Structural change typically alters albumin’s carrying capacity and therefore function. Examples include -
Fatty acids sites summary
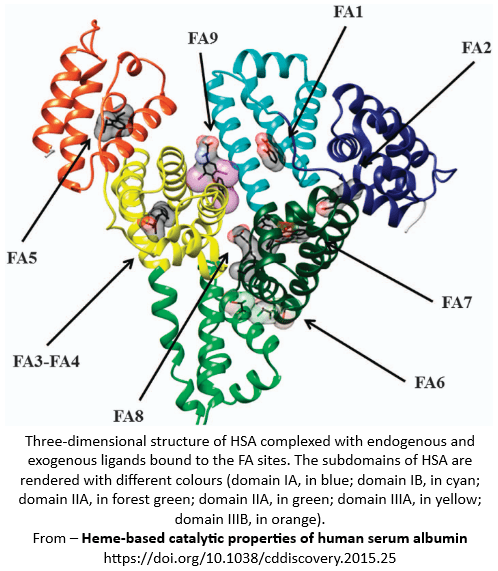
Nine fatty acids and their binding sites are currently identified.
FA1
Subdomain IB - aka the haem binding pocket, haem site, haem pocket FA1
Binds Haem, bilirubin, biliverdin, hemin
FA2
Between subdomains IA and IIA
Palmitate, stearate
FA3
Subdomain IIIA + IIB
α-tocopherol
Fatty acids with carbon groups -
- ≤ 12-14 - linearly
- ≥ 12-14 foldedFA4
Subdomain IIIA
α-tocopherol
Longer chain fatty acids - foldedFA5
Subdomain IIIB
Single fatty acids molecules
PalmitateFA6
Between subdomains IIA + IIB
Medium and long chain fatty acids
FA7
Subdomain IIA
α-linolenic acid
FA8
Between subdomains IA-IB-IIA and subdomains IIB-IIIA-IIIB.
Short chain fatty acids
FA9
Between subdomains IA-IB-IIA and subdomains IIB-IIIA-IIIB.
Short-chain fatty acids eg capric acid
Fatty acids and their binding sites by subdomain
Subdomain
Fatty acids
IA
IB
FA1
IIA
FA7
IIB
IIIA
FA3, FA4
IIIB
FA5
Domain
Fatty acids
IA-IIA interface
FA2
IIA-IIB interface
FA6
IA-IB-IIA and IIB-IIIA-IIIB interface
FA8, FA9
Clinical Concerns
We are accustomed to direct (linear) cause and effect interactions between pharmaceuticals and nutrition. For example, proton pump inhibitors alter gastric pH and consequently impair absorption of minerals such as magnesium, iron, calcium, etc.
The combination of structural flexibility, overlapping binding sites and allostery adds extra complexity to interactions between pharmaceuticals and nutrition. Allostery is comparable to thinking around corners!
Further, the structural flexibility, overlapping binding sites, allostery trifecta is an area where the clinical need-to-know is outpacing the research.
Clinical Questions
When you a review a person prescribed one or more drugs that utilize albumin as a carrier -
- would you question whether their higher serum fatty acids are altering albumin’s capacity to carry drugs and key nutrients?
Conclusions
Albumin and the fatty acids interactions affect which substances, including nutrients, can be carried by albumin at any given time.
Please read this as it is important …
The information in this article is provided to support Health Professionals. It is not an exhaustive protocol and Health Professionals are advised that adequate professional supervision is accessed to ensure that Duty of Care obligations with respect to safe administration of medicines is met for each consumer.