
Mechanisms and consequences
Drug administration for dysphagia is a fraught issue. Many pharmaceutical manufacturers recommend their products not be crushed, chewed, dissolved … which is fine if swallow capability is uncompromised. However, approximately 8% of the global population has dysphagia, with the elderly and disability sectors being particularly well represented. How can we safely administer prescribed medicines to those with dysphagia?
Dysphagia
Dysphagia encompasses all the factors that delay delivery of substances from the mouth to the stomach. The impairment can be acute or chronic, intermittent or persistent, mechanical (blockage) or motility (altered). Ultimately, dysphagia can negatively impact a person’s health, well-being, and quality of life.
Dysphagia is broadly divided into 2 types –
- oropharyngeal - problems in the mouth and pharynx. Typically includes difficulty in initiating a swallow, or in passing food through the mouth or throat;
- oesophageal - problems in the oesophageal body and esophagogastric junction. Typically includes structural or inflammatory abnormalities, or motility disorders.
IDDSI Guidelines
IDDSI is the International Dysphagia Diet Standardisation Initiative - https://www.iddsi.org/
Dietitians and Speech Pathologists created a working party to develop and implement a global Standard to describe current and desired textures of orally consumed substances. The purpose of this Standard is to enable safe swallowing in those with dysphagia.
IDDSI is now fully implemented which means the same terms and consistencies are applicable in any health service anywhere globally. This is an amazing achievement.
The ultimate aim is for all substances available for human oral consumption to be swallowed safely.
Drug administration
All consumed substances including medicines, must be administered in a form that is safe for individual intake. Alteration to medicine form, such as crushing, comes with a range of unintended consequences such as –
- altering drug absorption dynamics,
- altering drug stability,
- causing local (oesophageal, stomach) irritation,
- non-delivery of drug to desired site of action,
- increasing occupational health and safety risks to the person modifying the drug form. Harm can be caused due to skin exposure and/or inhalation,
- exposure of the consumer to unacceptable tastes. In this case compliance is the issue rather than effectiveness.
Consequently, some key questions that need to be asked include -
- can this drug be crushed or dispersed?
- can the capsule be opened?
- do any of this person’s currently prescribed medicines also compromise swallow function/safety?
- does this person’s swallow capability fluctuate during the day? Is their swallow capability safe whilst they are alert, and/or does it deteriorate with drowsiness/fatigue? And is this fluctuation consistent or intermittent?
- do the manufacturer’s recommended administration strategies comply with the IDDSI Standard?
- is this drug available in a safe-for-this person, format?
- are there onsite drug administration guidelines for this person with dysphagia?
- does this person still require this medicine?
Currently there is no regulatory requirement for dysphagia and enteral feeding guides to be included in the Product Information documents. Some of the pharmaceutical companies have differentiated between their customers (typically pharmacists and chemists) and their end-users (typically consumers of their products), and include this information in their Product Information documents.
Drug-induced dysphagia
Drug-induced dysphagia occurs when pharmaceutical products negatively impact the swallow reflex. Many commonly prescribed medicines have the potential to affect swallowing either –
- Directly – by impairing the swallow reflex;
- Indirectly – by causing drowsiness, dry mouth, oesophageal injury, or impairment to swallow muscle function, whereby the swallow reflex capability is compromised.
Key management/administration strategies
- Clarify whether the current medicines can be administered in alternate forms such as patches, pessaries, injectables. Note – there may be privacy, dignity and personal safety concerns with regular pessary administration.
- Always administer the drug with the same foodstuff to ensure consistency in drug effectiveness. Drug availability and effectiveness is altered by various foodstuffs. DO NOT grab the nearest foodstuff in the fridge or cupboard and mix the medicine into it. Over the years I have seen nurses administer drugs with apple sauce, honey, custard, jam, (not yet melted) ice cream, mashed potato, mashed pumpkin, pureed food of indeterminate content, and others!
- Record IDDSI level, and administration foodstuff on the drug chart and in each person’s Care Plan. A list of uncrushable drugs, and their alternatives, should also be attached to the drug chart as an easily-accessed resource.
- Ask your supplying pharmacist if they could provide a list of non-crushable drugs and their alternatives. If they can’t then ask if someone on light duties (WorkCover) can compile the list.
- If safe administration guidelines for a particular drug are unavailable, then ring the relevant drug company and ask for their recommendations. Document the advice given, note the person’s name and role, have someone else also listen to the conversation, and you both sign the entry in the Notes. This demonstrates you have sought “best possible advice” prior to initiating an action.
- Develop policies outlining drug administration for those with the various levels of dysphagia, and for those requiring enteral feeding. Submit the policies to your Medication Advisory Committee (MAC) for endorsement and distribution to all members.
- The policies should include if and when pill-crushing devices are to be used. Pill-crushing devices crush to the same standard every time whilst individuals crush to different standards. Variability in the crushing alters drug availability and effectiveness.
- Standardize the drug administration for dysphagia process and note on the drug chart where the guideline is filed. This is useful for new and casual staff. Standardizing the process limits potential harm.
- Include your standardized drug administration for dysphagia processes in each new staff member’s orientation program. Ensure skills and understanding are demonstrated before allowing new staff to administer any drugs to those with dysphagia.
- Clarify drooling management strategies if there is concurrent dysphagia and medication administration.
- For those services that may not have a MAC, there are some options worth considering such as -
- attending some MAC meetings at your local RACF (Residential Aged Care Facility) prior to setting up your own MAC,
- canvassing the possibility of submitting some policies to one of your local RACF MAC meetings. This will enable medical, pharmaceutical, and hopefully nutrition and speech pathology, insights;
- organizing joint RACF MAC meetings X times per year;
Your policies and procedures both increase the likelihood and emphasize the importance of correct drug administration. In Victoria, death by choking automatically triggers a Coronial investigation. This regulatory requirement has significantly improved drug administration compliance.
Clinical concerns
How many people in your care diagnosed with dysphagia are prescribed medicines that include dysphagia as a side effect? If the prescribed medicines were changed to alternatives that did not include dysphagia as a side effect, then is it likely will the dysphagia resolve?
Do you, or have you heard any doctor, pharmacist, nurse or other health professional, question whether other prescribed medicines are contributing to, or exacerbating the person’s dysphagia? I haven’t.
How compliant is drug administration with the IDDSI Guidelines? Is IDDSI compliance with drug administration a standard inclusion in all Care audits?
Managing dysphagia, and especially drug-induced dysphagia, does not seem to be integrated into the daily clinical practice of relevant clinicians. Perhaps it is time for all the relevant professional bodies, universities and other training institutions to address this profoundly relevant clinical oversight.
Clinical Questions
What actions will you initiate as you a review a person whose diagnoses includes dysphagia, will you -
- identify each of the prescribed medicines that directly causes dysphagia? And question whether any can be changed to an equivalent that does not cause dysphagia?
- clarify and monitor whether the dysphagia fluctuates?
- clarify whether there are appropriate, IDDSI-compliant, drug administration guidelines onsite?
- audit, or recommend regular auditing of current medicine-modification strategies for compliance with IDDSI guidelines?
- attend a MAC meeting and possibly become a member?
Conclusions
Drug administration for dysphagia raises many concerns in relation to both safe administration, and compliance with IDDSI guidelines.
Useful resources
Title
Availability
Don't Rush to Crush
Australia’s essential guide to safely administering oral medicines to those with dysphagia, and those requiring enteral feeding.
Available in the AdPha Bookshop or online through eMIMS, MIMS Online (1800 800 629) and AusDI (1300 118 120).
https://adpha.au/publications-resources/drtcMedication Management in Residential Aged Care Facilities Guiding Principles.
Medication Management in the Community Guiding Principles.
Role of a Medication Advisory Committee User Guide (2022)
Swallowing safety of oral liquid medications: assessment using the International Dysphagia Diet Standardisation Initiative framework (2022)
Case study
The comments refer to the drug-nutrient, drug-food, and PharmacoNutrition effects only.
Data summary
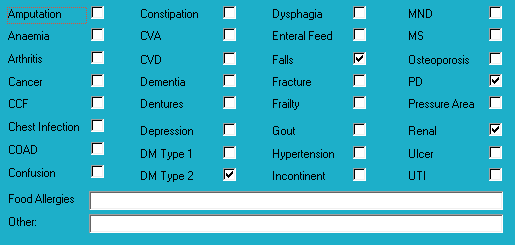
Medical history with nutritional aspect
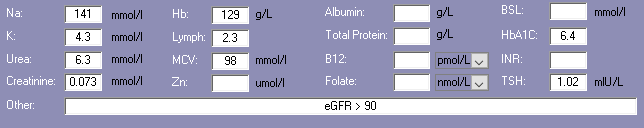
Biochemistry with nutritional aspect
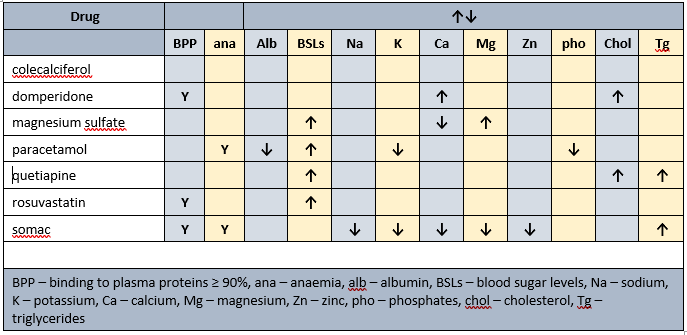
Prescribed medications side effects profile - biochemistry
Prescribed medications side effects profile
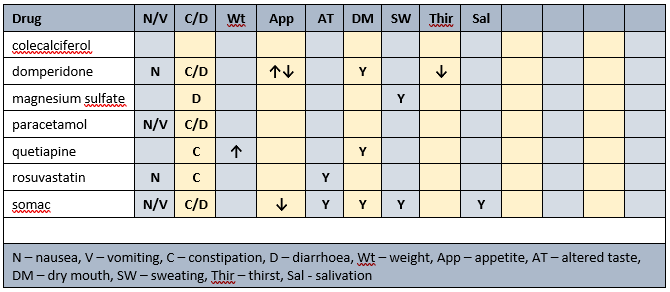
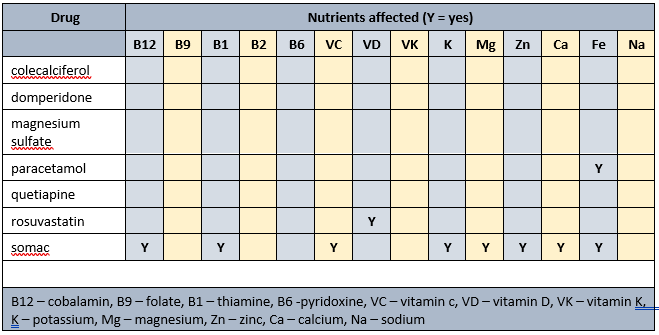
Prescribed medications affected nutrients profile
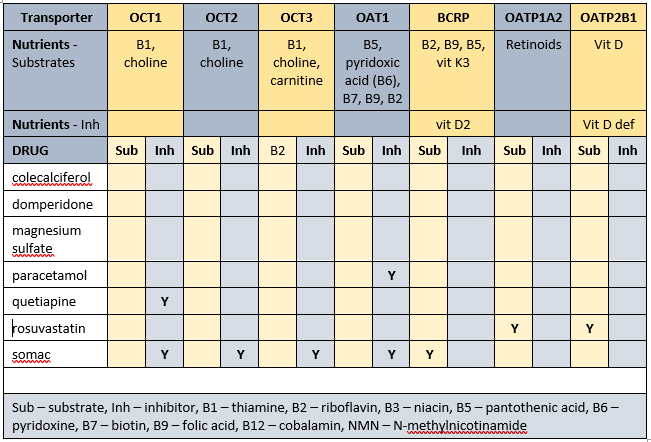
Transporter-mediated interactions and nutrients matrix
Biochemistry
Relatively recent available relevant biochemistry indicates -
- low Hb - associated with increased risk of falls, and poor appetite. Currently prescribed paracetamol and somac, both of which impair iron availability;
- elevated MCV - advisable to check B12 levels. Currently prescribed somac which decreases B12 absorption.
Advisable to check plasma proteins (albumin, total proteins) as they are the primary transporters for 3 of the prescribed drugs and hypoproteinaemia may alter their effects.
Glycaemia
BSLs (May-Jun)
- before breakfast - 5.0-9.8; recommended range 4-6
- daily range - 5.0-15.9; recommended range - 4-10
- tested daily bd
- reportable limits: < 4 and > 20
- recent HbA1c indicates acceptable overall glycaemic control
Diabetes drugs
- novomix 30 has a time to onset of 5-15 minutes, variable time to peak, and duration of 10-16 hours
Diabetes drugs coverage
- before breakfast BSLs - minimal, if any, coverage from previous morning's novomix, or previous evening's novomix
- before evening meal BSLs - minimal, if any, coverage from previous evening's novomix; covered by current morning's novomix
Currently prescribed 4 medications that alter glycaemia.
PharmacoNutrition
Currently prescribed vita-D cap (1/day). Advisable to check vitamin D levels and if low then review current vitamin D management strategy.
Currently prescribed mag-sup (1 tab/day) which provides 37.4 mg elemental magnesium per tab; men require 420 mg elemental magnesium/day. Also prescribed Somac which impairs magnesium absorption. Magnesium supplement commenced 5 years ago. Advisable to check magnesium status and if less than 0.80 pmol/L then advisable to review effectiveness of current magnesium intervention and consider alternate products, and administering prior to somac administration.
Concurrent ingestion of paracetamol and iron resulted in increased rate of iron absorption and decreased absorption of paracetamol. The authors advise different administration times from each other for the drug and iron.
Dietary levels of caffeine intake in conjunction with paracetamol inhibit antinocieception. Somac decreases B12, vitamin C, magnesium, zinc and iron absorption, may decrease calcium absorption, and decreases thiamine availability.
Statins interfere early in the cholesterol metabolic pathway and consequently decrease -
- conversion of sun to vitamin D - vitamin D intervention recommended;
- production of CoQ10 - important in cellular energy production; CoQ10 intervention recommended;
- DHEA production - low DHEA associated with increased risk of metabolic syndrome; intervention recommended.
Membrane transporters
Some of the identified membrane transporters alter the absorption and/or organ and cellular uptake of a range of nutrients. Inhibition of membrane transporters means blood test results may be unreliable. To clarify nutrient status advisable to conduct blood tests at least one hour before administration of relevant prescribed medicines. A concurrent detailed Diet History is also essential to corroborate adequacy of intake of all affected nutrients. Further, all affected nutrients to be monitored on a regular basis ie at least annually.
Nutrients that are affected by Mr ADI’s prescribed medications include -
- substrates – thiamine, riboflavin, pantothenate, pyridoxine, biotin, folate, vitamin D, vitamin K3, choline, carnitine, retinoids;
- inhibitors – vitamin D, vitamin D deficiency.
The duration of drug inhibition of transporters currently remains unknown.
Bowel management
- no regular intervention prescribed;
- oral PRN aperient prescribed;
- no Nurse Initiated interventions administered.
Observations
Mr ADI is a pale man who was sitting in his room watching TV and rubbing his right arm when I went to speak to him - he told me his (R) arm was quite painful and was due to a recent fall. Mr ADI also told me his sleeping tablets are no longer effective.
Mr ADI‘s overall condition seems to have deteriorated in the last 12 months.
Mr ADI has remained remarkably weight stable for the last year.
PharmacoNutrition comments
Mr ADI has been prescribed a proton pump inhibitor for the last 6 years, and possibly before then. Evidence increasingly indicates that longterm (3+ years) proton pump inhibitor prescription is associated with -
- altered gut microbiome;
- increased risk of food sensitivities at a level of peanut allergy, due to partial protein digestion;
- increased risk of coeliac disease due to partial protein digestion;
- increased risk of scurvy;
- generalised malnutrition due to impaired absorption of a range of nutrients such as B12, vitamin C, magnesium, zinc, iron, etc;
- altered gastric pH which reduces absorption dynamics of a range of drugs and nutrients. Altered drug availability is relatively easily identified however reduced nutrient absorption is rarely identified due to the non-specific nature of their signs and symptoms.
Consequently advisable to reconsider reviewing current proton pump inhibitor prescription and consider
- whether proton pump inhibitor prescription is still required
- if suppression of gastric acidity is still required then could it be managed with an H2 antagonist such as ranitidine (there is a general belief that they cause less nutritional harm than proton pump inhibitors)
Mitochondrial dysfunction is now being linked to a number of disorders including heart failure, cardiovascular diseases, diabetes, neurodegenerative diseases (MS, Huntingtons, Parkinsons, MND, Alzheimers, glaucoma), ageing, mitochondrial diseases, retinis pigmentosa, CVA, epilepsy, cardiomyopathy, autism, muscular dystrophy, atypical learning disabilities, fibromyalgia, Chronic Fatigue Syndrome, Developmental Delay, Cerebral Palsy, bipolar disorder, major depression disorder, schizophrenia, pulmonary fibrosis, and likely others. Statins are contra-indicated in the presence of dysfunctional mitochondria.
There is an interrelationship between glycaemic control and lipid control. Since Mr ADI seemingly has overall good glycaemic control, and the contra-indication for statin prescription in the presence of dysfunctional mitochondria, advisable to review necessity for continued prescription of rosuvastatin.
Mr ADI’s diagnoses include Parkinson’s Disease (PD). Nutritional factors associated with PD include -
- vitamin D – adequate vitamin D status slows/delays disease progression. Currently prescribed colecalciferol (to increase vitamin D levels) and rosuvastatin (which decreases vitamin D levels) therefore advisable to clarify current vitamin D status and if low then review adequacy of current vitamin D intervention;
- B12 – important in myelin maintenance and production. Currently prescribed somac which decreases B12 absorption. Advisable to clarify B12 status and if low then consider an intervention;
- thiamine – is important in many aspects of mitochondrial, astrocytic and neuronal function. Currently prescribed somac which directly impairs thiamine availability, and indirectly impairs thiamine availability by decreasing magnesium absorption.
- vitamin C – modifies production of Reactive Substances and inhibits oxidative stress; is important in mitochondrial, astrocytic and neuronal function, Currently prescribed somac which directly impairs vitamin C availability, and indirectly impairs vitamin C availability by decreasing magnesium absorption.
Mr ADI’s diagnoses include diabetes. Nutritional factors associated with altered glycaemia include -
Thiamine. Important in carbohydrate metabolism. Currently prescribed somac which impairs availability both directly and indirectly. Advisable to clarify thiamine status and if low then intervention recommended;
Vitamin D. Increases the number of insulin receptors on the cells. Currently prescribed rosuvastatin therefore advisable to clarify vitamin D status and if low then intervention recommended;
Iron. The beta cells in the pancreas do not tolerate inadequate or excessive iron intake and decrease insulin production as a consequence. Currently prescribed paracetamol and somac therefore advisable to clarify iron and copper levels and initiate an intervention if either are low;
Magnesium. Is important for activation of thiamine, vitamin C and vitamin D, and regulation of the IR/IRS/PI3K/PDK/Akt/GLUT4 pathway. Currently prescribed magnesium sulfate (to increase magnesium levels) and somac (which decreases magnesium absorption}. Advisable to clarify magnesium status and if low then advisable to review adequacy of current magnesium intervention and timing of its administration in relation to somac;
Mr ADI is prescribed 4 drugs that are associated with drug-induced hypergycaemia, and may be contributing factors to Mr ADI’s history of variable glycaemic control.
Mr ADI’s diagnoses include falls. Nutritional factors that may be useful to ensure within acceptable ranges include –
- vitamin D – increasing vitamin D intake increases muscle strength and decreases falls. Currently prescribed colecalciferol (to increase levels) and somac (which decreases levels by decreasing magnesium absorption). Advisable to clarify vitamin D status, and if low then review adequacy of current intervention;
- B12 - is important in the righting reflex when a person stumbles. Currently prescribed somac which decreases B12 absorption therefore advisable to check status;
- zinc – can decrease food intake through altered sense of taste and poor appetite, and consequently reduced muscle mass. Currently prescribed somac which decreases zinc absorption therefore advisable to clarify status;
- magnesium - magnesium is important in vitamin D activation, de novo carnitine production, and muscle function, amongst other functions. Currently prescribed magnesium sulfate (to increase magnesium levels) and somac (which decreases magnesium absorption}. Advisable to clarify magnesium status and if low then advisable to review adequacy of current magnesium intervention and timing of its administration in relation to somac;
- thiamine - is important in balance and position sense. Currently prescribed somac which indirectly impairs thiamine status by decreasing magnesium absorption. Advisable to monitor thiamine status;
- carnitine - carnitine is both absorbed and produced de novo, and is important in a range of muscle functions. Magnesium is important in de novo carnitine production. Currently prescribed magnesium sulfate (to increase magnesium levels) and somac (which decreases magnesium absorption}. Advisable to clarify magnesium status and if low then advisable to review adequacy of current magnesium intervention and timing of its administration in relation to somac.
What else would you include?
Please read this as it is important …
The information in this article is provided to support Health Professionals. It is not an exhaustive protocol and Health Professionals are advised that adequate professional supervision is accessed to ensure that Duty of Care obligations with respect to safe administration of medicines is met for each consumer.

